Defibrillators Market Accelerates with FDA Nod to Element Science’s Jewel® Patch-WCD, Ushering in a New Era of Wearable Cardiac Care | DelveInsight
DelveInsight’s Defibrillators Market Insightsreport offers current and forecast market analysis, leading defibrillator companies’ market shares, emerging challenges, market drivers, barriers, trends, and profiles of key defibrillator companies shaping the competitive landscape.
Key Takeaways from the Defibrillator Market Report
• In May 2025, Element Science received FDA approval for its Jewel® Patch Wearable Cardioverter Defibrillator (Patch-WCD), marking a major milestone in cardiac care. Designed for patients at temporary high risk of sudden cardiac arrest, the device offers a discreet, life-saving solution. It was previously granted CE and UKCA certifications in January 2024.
• In April 2025, Medtronic (NYSE: MDT) received FDA approval for its OmniaSecure™ defibrillation lead—the world’s smallest at just 4.7 French (1.6mm). Built on the trusted SelectSecure™ Model 3830 and delivered via catheter, the lead is designed for precise placement in the right ventricle to treat VT/VF and bradyarrhythmias. It is approved for adults and adolescents aged 12+, including patients with smaller anatomies.
• In June 2024, Stryker announced the launch of its latest product in the monitor/defibrillator space, the LIFEPAK 35 monitor/defibrillator. The device was designed to support life-saving teams by providing real-time access to critical patient information. Built on an intuitive, modern platform, the LIFEPAK 35 aimed to advance patient care. It featured a slim, light, and ergonomic design, along with a large, intuitive touchscreen.
• In June 2024, Elutia Inc., a pioneer in drug-eluting biomatrix products, announced that Antibiotic-Eluting BioEnvelope, EluPro (previously known as CanGaroo during development), received clearance from the U.S. Food and Drug Administration (FDA). Designed to prevent post-operative complications for devices like pacemakers and defibrillators, EluPro integrated potent antibiotic therapy with advanced tissue engineering. This BioEnvelope was created to gradually regenerate into a protective pocket of the patient’s own tissue over time.
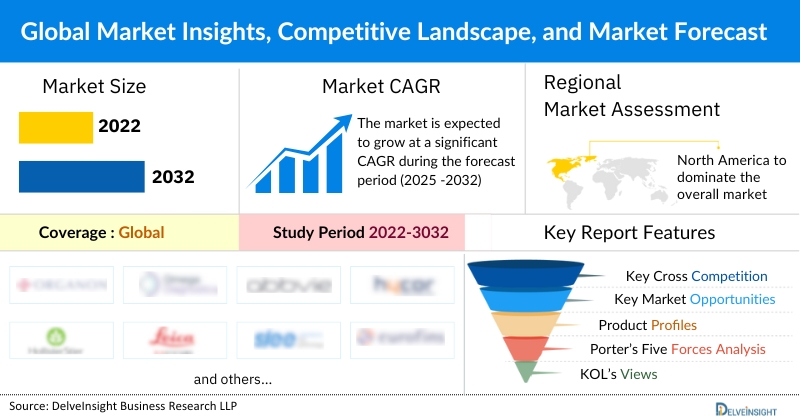
• The defibrillators market, valued at USD 10.19 billion in 2023, is projected to grow at a 3.11% CAGR, reaching USD 12.24 billion by 2032.
• As per DelveInsight estimates, North America is anticipated to dominate the global defibrillator market during the forecast period.
• Notable defibrillator companies such as Medtronic, Abbott, Boston Scientific Corporation, Stryker, Koninklijke Philips N.V., Zoll Medical Corporation, Biotronik, Nihon Kohden Corporation, and several others are currently operating in the defibrillator market.
To read more about the latest highlights related to the defibrillator market, get a snapshot of the key highlights entailed in the Global Defibrillator Market Report
Defibrillator Overview
A defibrillator is a life-saving medical device used to restore a normal heartbeat by delivering an electric shock to the heart in cases of sudden cardiac arrest (SCA) or life-threatening arrhythmias like ventricular fibrillation and pulseless ventricular tachycardia. These devices are critical in emergency cardiac care and are commonly found in hospitals, ambulances, public places, and increasingly in homes.
There are several types of defibrillators, including:
-
Automated External Defibrillators (AEDs): Designed for public use by laypersons with minimal training, AEDs analyze heart rhythm and deliver shocks if needed.
-
Implantable Cardioverter Defibrillators (ICDs): Surgically implanted in high-risk patients, these continuously monitor heart rhythms and deliver shocks when abnormal rhythms are detected.
-
Wearable Cardioverter Defibrillators (WCDs): External, non-invasive devices for patients temporarily at risk of cardiac arrest.
-
Manual External Defibrillators: Used by healthcare professionals in clinical settings for advanced resuscitation.
Technological advancements are making defibrillators more portable, intelligent, and easier to use, expanding their adoption across healthcare and non-clinical environments. Their growing availability and public awareness are key to improving survival rates in cardiac emergencies.
Defibrillator Market Insights
The defibrillators market in North America is expected to dominate and maintain its lead through 2032, driven by the high prevalence of coronary artery disease, myocardial infarctions, and other cardiovascular conditions. As per recent data, over 20.5 million Americans live with CAD, and approximately 805K heart attacks occur annually in the U.S., highlighting the critical need for defibrillators. In Canada, 2.4 million people were affected by heart disease in 2022. The growing burden of cardiac conditions, coupled with increasing awareness, widespread device availability, and regulatory advancements, such as the FDA approval of Kestra’s ASSURE® Wearable Defibrillator, are fueling the growth of the defibrillators market in the region.
To know more about why North America is leading the market growth in the defibrillator market, get a snapshot of the Defibrillator Market Outlook
Defibrillator Market Dynamics
According to the World Heart Report 2023, over 500 million people were living with cardiovascular diseases (CVDs) globally in 2021. The American Heart Association (2023) estimates that by 2030, over 12 million people will have atrial fibrillation worldwide, while the British Heart Foundation (2024) reports around 200 million people living with coronary artery disease (CAD).
Defibrillators are vital in managing CVDs, particularly for conditions like atrial fibrillation and CAD. They deliver life-saving electric shocks during sudden cardiac arrest (SCA), helping restore normal heart rhythm and improve survival chances. Their use is especially critical among aging populations, as older adults face a higher risk of cardiac events. The World Health Organization projects that by 2030, 1 in 6 people globally will be over 60 years old, further increasing demand for emergency cardiac care.
Market growth is also driven by regulatory approvals and innovations. For instance, Element Science received CE and U.K. approval in 2024 for its wearable cardioverter defibrillator, and Medtronic’s Aurora EV-ICD™ and Epsila EV™ systems received FDA approval in 2023 for treating life-threatening arrhythmias.
However, potential side effects, such as skin burns from shocks, pose challenges to market expansion.
Defibrillator Market Drivers:
• Increasing global prevalence of conditions like sudden cardiac arrest, atrial fibrillation, and coronary artery disease is driving demand for defibrillators as essential life-saving devices.
• Innovation in wearable and implantable defibrillators, along with regulatory approvals (e.g., Medtronic’s EV-ICD™ and Element Science’s patch defibrillator), is boosting adoption and market growth.
Defibrillator Market Barriers:
• Risks such as burns, skin damage, and discomfort associated with electric shocks can deter patient acceptance and device usage.
• The high cost of advanced defibrillators and limited healthcare infrastructure in developing regions can restrict market penetration.
Get a sneak peek at the defibrillator market dynamics @ https://www.delveinsight.com/report-store/defibrillator-market
Coverage: Global
Study Period: 2022 to 2032
Key Defibrillator Companies: Medtronic, Abbott, Boston Scientific Corporation, Stryker, Koninklijke Philips N.V., Zoll Medical Corporation, Biotronik, Nihon Kohden Corporation, and others.
Biopsy Devices Market Segmentation
Market Segmentation By Product Type: Implantable Cardioverter Defibrillator [Single Chamber ICD, Dual Chamber ICD, CRT-D, Subcutaneous ICD (S-ICD)], and External Defibrillator [Semi-Automated, Automated, and Wearable].
Market Segmentation By End User: Hospitals, Ambulatory Surgical Centers, Homecare Settings, and Others
Market Segmentation By Geography: North America, Europe, Asia-Pacific, and Rest of the World
Which MedTech innovators are shaping the future of the defibrillator market? Explore the leading Defibrillator Companies Driving Innovation.
Table of Contents
1. Electrophysiology Devices Market Report Introduction
2. Electrophysiology Devices Market Executive Summary
3. Competitive Landscape
4. Regulatory Analysis
5. Electrophysiology Devices Market Key Factors Analysis
6. Electrophysiology Devices Market Porter’s Five Forces Analysis
7. Electrophysiology Devices Market Layout
8. Electrophysiology Devices Market Company and Product Profiles
9. KOL Views
10. Project Approach
11. About DelveInsight
12. Disclaimer & Contact Us
Report Features
DelveInsight’s Defibrillators Market Report delivers a detailed analysis of the current market landscape and future growth potential. It highlights recent product innovations, strategic mergers, acquisitions, and partnerships that are shaping the competitive environment. The report identifies key companies leading the market, top-performing segments, and high-potential regions offering opportunities for expansion. It also outlines areas where emerging players can establish a strong presence. This report is essential for defibrillator manufacturers, research firms, industry associations, government bodies, investors, distributors, and end-users looking to stay informed about the latest technological advancements and market trends.
About DelveInsight
DelveInsight is a premier healthcare business consultant and market research firm, specializing in life sciences. We empower pharmaceutical companies with comprehensive end-to-end solutions designed to enhance performance and drive growth.
Our expert healthcare consulting services offer in-depth market analysis, helping businesses accelerate growth and navigate challenges with actionable, results-driven strategies.
Media Contact
Company Name: DelveInsight
Contact Person: Jatin Vimal
Email: Send Email
Phone: +14699457679
Address:304 S. Jones Blvd #2432
City: Las Vegas
State: Nevada
Country: United States
Website: https://www.delveinsight.com/